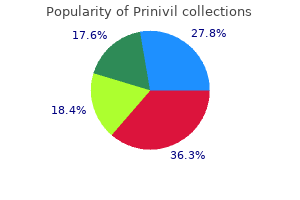
Prinivil
"Cheap prinivil 10 mg mastercard, blood pressure medication with diabetes".
By: L. Brant, M.B.A., M.D.
Co-Director, Wayne State University School of Medicine
Mechanism of Action Metolazone is a thiazide-type diuretic whose primary site of action is the distal convoluted tubule and whose secondary site of action is the proximal tubule prehypertension ppt order prinivil on line amex. Diuretic Medications 131 these regions heart attack zip prinivil 2.5 mg line, metolazone inhibits sodium reabsorption blood pressure headaches purchase prinivil overnight delivery, causing increased excretion of sodium and water as well as potassium and hydrogen ions. Oral absorption of the drug is dependent on the prepa- ration used, and protein binding is 95%. The half-life is 6 to 20 hours and 70 to 95% of the drug is eliminated unchanged in the urine. Hypokalemia, hyponatremia, hypochloremia, metabolic alkalosis, hyperglycemia, thrombocy- topenia, leukopenia, aplastic anemia, and hyperuricemia have also been reported. Poisoning Information Metolazone overdose is commonly characterized by orthostatic hypotension, diz- ziness, drowsiness, fainting, and volume depletion. Treatment is supportive and symptomatic and replacement of fluid and electrolyte losses may be necessary. There are also increased potassium losses with steroids and amphotericin B; and increased hypersensitivity reactions to allopurinol. There is increased inci- dence of digoxin toxicity caused by hypokalemia and hypomagnesemia. It increases the excretion of sodium, chloride, and water and inhibits the excretion of potassium and hydro- gen. Neonates: Diuretic: 1 to 3 mg/kg/day divided every 12 to 24 hours Children: Diuretic, hypertension: 1. The protein binding of the drug ranges from 91 to 98%, with hepatic metabolism to multiple metabolites, including the active agent, canrenone. The half-life of spironolactone is 78 to 84 minutes and the half-life of canrenone is 13 to 24 hours. Diuretic Medications 133 Use with caution in patients with decreased renal function, hyponatremia, dehydration, or reduced hepatic function. Adverse reactions associated with spironolactone include hyperkalemia, dehydration, hyponatremia, hyperchloremic metabolic alkalosis, headaches, fever, diarrhea, vomiting, nausea, lethargy, rash, anorexia, gynecomastia (in males), amenorrhea, agranulocytosis, and decreased renal function. Poisoning Information Symptoms of spironolactone overdose include lethargy, fatigue, drowsi- ness, dizziness, confusion, nausea, and vomiting. Dehydration, electrolyte imbalance, and severe hyperkalemia may occur with large doses. Spironolactone use may decrease clearance of digoxin; may cause a decreased response to norepinephrine; and may decrease the effects of oral anticoagulants. Mechanism of Action Amiloride inhibits sodium-potassium ion exchange in the distal convoluted tubule by inhibiting cellular sodium transport mechanisms and inhibits hydro- gen ion secretion; its diuretic activity is not dependent on aldosterone. Kazmerski Pharmacokinetics Amiloride has an onset of action of 2 hours and a duration of 24 hours. The half-life in normal renal function is 6 to 9 hours and up to 144 hours in severe renal disease. Carbonic Anhydrase Inhibitors Acetazolamide Indication Acetazolamide is used to reduce intraocular pressure in glaucoma and as a diuretic. Diuretic Medications 135 Mechanism of Action As a diuretic, acetazolamide initiates competitive, reversible inhibition of car- bonic anhydrase, which results in increased renal excretion of sodium, potas- sium, bicarbonate, and water. Absorption of acetazolamide is dose dependent, and acetazolamide distributes into erythro- cytes and the kidneys. Use with caution in patients with chronic obstructive pulmonary disease, respiratory acidosis, gout, and diabetes mellitus; reduce dosage in patients with renal dysfunction. Common side effects of acetazolamide include cyanosis, drowsiness, fever, seizures, dizziness, depression, rash, photosensitivity, vertigo, hypokalemia, hyperchloremic metabolic acidosis, hyperglycemia, nausea, vomiting, black 136 D. Kazmerski stools, polyuria, muscle weakness, anorexia, cholestatic jaundice, hepatic insufficiency, and hyperpnea. Poisoning Information Symptoms of acetazolamide overdose include drowsiness, nausea, vomiting, confusion, tachycardia, sweating, dizziness, convulsions, tingling of lips and tongue, and low blood sugar.
These apparent changes in spectrum are exclusively due to various characteristic features pulse blood pressure relationship buy cheap prinivil, namely : (a) Nature of the solvent blood pressure 75 over 55 order prinivil 5mg visa, (b) Nature of the absorption band blood pressure medication vasodilators cheap prinivil american express, and (c) Nature of the solute. Interestingly, inclusion of readily polarizable atoms do exert an effect likewise to lengthening a con- jugated chain. It is pertinent to mention here that there are a plethora of commercially available spectrophotometers of varying design i. Evidently, it is practically impossible to describe either all or even a major fraction of, the various spectrophotometers available. Therefore, in this particular section the following two types of spectrophotometers shall be discussed briefly : (a) Single-beam Spectrophotometer, and (b) Double-beam Spectrophotometer. The wavelength dial on a spectrophotometer is adjusted to a specific value, but the radiation leaving the exit-slit is found to be rarely monochromatic. The slit-entrance mirror subsequently deflects the beam through the adjustable slit (D) and into the monochromator to the collimator mirror (E). As a result the light falling on the collimator mirror is rendered parallel and reflected to the prism (F), where it undergoes refraction. The back surface of the prism is aluminized, so that the light refracted at the first surface is reflected back through the prism, undergoing further refraction as it emerges. The desired wavelength of light is selected by rotating the wave- length selector fixed on top of the monochromator case. The spectrum from the prism is directed back to the collimating mirror which centres the chosen wavelength of light on the slit and the sample (G). Light passing through the sample strikes the phototube (H), causing a voltage to appear across a load-resistor. The voltage is duly amplified and registered on either the strip-chart recorder or the null-meter. The Milton Roy Spectronic(R)-20 definitely provides a low-cost and easy to operate instrument, that is still capable of achieving absorbance readings accurate to ± 1 or 2%. A computer system has also been provided to enable automatic spectrochemical measurements and perform calculations simultaneously. It could be accomplished by the help of the following two cardinal modifications, namely : (a) Need for a continuous change in wavelength so that light through the blank and through the sample may be monitored continuously, and (b) Measurements done with a recording spectrophotometer. The above two modifications have been duly incorporated in a double-beam spectrophotometer. In fact, the source beam is usually split in two different manners, namely : (a) Separated in Space : In this instance, the source beam is split between the sample cell-path and the reference cell-path, and finally detected by two diode detectors. Here, the two detectors should be adequately matched so that no changes occur relative to each other during the measurements, (b) Separated in Time : In this case, the source beam is split with the help of an optical chopper which permits the source beam to alternate between the sample cell-path and the reference cell- path. Here, the source should be stable enough so that no changes take place in the radiant energy during the chopping time. Keeping in view, this specific, rigid and stringent requirement, the separation-in-space method is found to be normally of lower precision and accuracy than the separation-in time-method. Evidently, the optical choppers are quite expensive, and therefore, the instrument manufacturers very often utilize the separation-in-space method for the routine measurement spectrophotometers. However, the most sophisticated double-beam spectrophotometer is usually pretty expensive by vir- tue of the following facts, namely : (i) Greater operating stability, (ii) Rapid speed compared to single-beam instruments, (iii) Complicated optical system involved, and (iv) Recording device for recording absorbance Vs wavelength. These instruments are mostly based on microcomputer-controlled devices with built-in recorder to accom- plish faster speed and greater operating stability. Extinction is solely dependent upon the following two factors, namely : (a) Concentration of the absorbing substance present in the solution, and (b) Thickness of the absorbing layer taken for measurement. Bearing in mind the ease in calculations and also the convenience of reference, the extinction of a 1-cm layer of a 1% w/v solution is usually recommended in most of the official compendia (i. This particular property is the basis for most assay methods included in pharmacopoeia that are absolutely free from interfering materials, besides being utilized for identifying substances. In actual practice, where a test or an assay recommends the usage of a Reference Substance, the spectrophotometric measurements are always performed first with the solution prepared from the Reference Substance by the directions provided in the specific monograph and then with the corresponding solution prepared from the substance under examination. Nevertheless, the second measurement must be done immediately after the first, by employing the same cell and the same instrumental parameters. Importantly, when a double bond recording instrument is being employed the solvent cell is always placed in the reference beam. Particular care must be taken to employ solvents free from contaminants absorbing in the specific spectral region being used.
As the prevalence of diabetes increases rapidly in the developing world blood pressure 8555 order prinivil 10mg with mastercard, new prehypertension how to treat buy prinivil uk, loosely regulated markets attract potential counterfeiters blood pressure 8959 prinivil 5mg with amex. India is home to more than 50 million diabetics, more than any other country, and the number is expected to increase dramatically over the coming years (World Diabetes Foundation, 2013). In 2007, not long after the frst bad test strips appeared in the United States, there were ap- proximately 40. As the chronic disease burden in- creases in developing countries, falsifed and substandard versions of the expensive products used to treat them pose new risks. Maintenance medication for cardiovascular disease is a vulnerable target for fraud, but the need for these drugs is still unmet in much of the world (Gaziano, 2007). In developing countries, there has been a greater emphasis on controlling infectious disease, especially the infectious diseases of childhood. Considerable research indicates that the anti-infective drugs used to do this are often compromised in poor countries. A more recent survey in Egypt, Jordan, Lebanon, and Saudi Arabia found more than half of antibiotics sampled to be substandard (Kyriacos et al. A similar survey in Burma uncovered substandard drugs in 16 per- cent of amoxicillin and 13 percent of ampicillin samples (Wondemagegnehu, 1999). More recently, a survey of amoxicillin in the capital of Papua New Guinea found all samples outside of pharmacopeial specifcations; 14 per- cent had undetectable levels of active ingredient (Nair et al. In most low- and middle-income countries β-lactam antibiotics, an inexpensive and widely available class of drugs that includes penicillin and amoxicillin, are the frst-line treatment for dozens of bacterial infections, including scarlet fever, pneumonia, and respiratory and urinary tract infec- tions (Byarugaba, 2004). Alone it ac- counts for as much as 12 percent of all child deaths worldwide (O’Brien et al. This will remain the best strategy un- til the pneumococcal conjugate vaccine becomes more widely available. The treatment of pneumonia and other devastating bacterial infections depends on effective antibiotic supply. No research to date has attempted to quan- tify the proportion of child deaths attributable to falsifed and substandard medicines, but Table 2-1 presents the most common causes of child death and links them to verifed reports of substandard medicines. Chapter 5 describes the medicines supply chain in developing countries; in Copyright © National Academy of Sciences. If antibiotics are some of the oldest and most widely used medicines in the world, antiretrovirals are their opposites: new medicines, prescribed in complicated regimes, to a relatively small segment of the population. In 1995, during a meningitis epidemic, about 60,000 Nigeriens were injected with water disguised as meningitis vac- cine (Cockburn, 2005). More recently, in China, substandard hepatitis B and rabies vaccines killed or sickened about 100 babies (Jia and Carey, 2011). Precise infor- mation regarding the event is scarce due to the Chinese government’s denial of a connection between the vaccines and the incidents as well as its control over the Chinese media. According to the Associated Press, the original article in the China Economic Times that exposed the scan- dal stated that four children who died never had a precise diagnosis, but sufered from fevers and convulsions before their deaths; others who became ill were later diagnosed with encephalitis, among other conditions, and some sufered permanent damage (Associated Press, 2010a). About 200,000 doses of substandard rabies vaccine circulated in Jiangsu province in 2010 before a manufacturer recall (Associated Press, 2010b). These vaccines, like the falsifed meningitis vaccine used in Niger, convey no immunity to the patient. When herd immunity is an important result of vaccination, there is no such beneft to society. Assuming the patients survive injection with nonsterile, unidentifed liquids, they are still at risk for death from the disease they were not inoculated against. In September 2011, falsifed and substandard versions of the triple combination therapy Zidolam-N surfaced in Kenya, many samples molding and crumbling in the packages (Taylor, 2011). A year later, in Tanzania, the regulatory authority uncovered falsifed anti- retrovirals at a district hospital (Athumani, 2012). As their viral loads increase, these patients are also more likely to transmit the infection, impeding efforts to control the virus. Although data suggesting compromised antiretroviral drug quality are mixed, there is substantial evidence, presented in Chapter 3, that antima- larials are often of poor quality. Substandard and falsifed malaria drugs are especially common in malaria-endemic parts of Africa and Asia. In 2003, substandard sulfadoxine-pyrimethamine was used to treat a malaria epidemic in northwest Pakistan refugee camps (Leslie et al.
Syndromes
- Seizures
- Increased BUN and creatinine blood levels
- The name of the product (ingredients and strengths if known)
- Arterial blood gas, which measures oxygen and carbon dioxide levels in the blood
- Walking aids to help with balance and prevent falls
- Breathing difficulty
- Pfeiffer syndrome
- Weakness
Examples : Magnesium Trisilicate blood pressure medication makes me feel weird purchase prinivil cheap, Bentonite blood pressure yoga poses purchase prinivil no prescription, Barium Sulphate arrhythmia ppt order prinivil online from canada, Light and Heavy Kaolin. The latter being volatile in nature can be separated by distillation 3 from remaining metallic salts and the distillate examined in the normal manner. Limit Test for Iron Theory : The limit test for Iron is based on the reaction between iron and thioglycollic acid in a medium buffered with ammonium citrate to give a purple colour, which is subsequently compared with the standard colour obtained with a known amount of iron (0. Ferrous thioglycollate is aco-ordination compound that attributes the purple colour ; besides thioglycollic acid converts the entire Fe3+ into Fe2+. As these two acid radical impurities are found in abundance due to contamination, the Pharmaco- poeia categorically stipulates limit tests for them which after due minor modifications are applicable to a number of pharmaceutical substances. In addition to the above two commonly found impurities, there are a number of other acid radical impurities which exist in pharmaceutical substances, namely : arsenate, carbonate, cyanide, nitrate, oxalate, phosphate and silicate. The opalescence produced is not greater than the standard opalescence, when viewed transversely. A few typical examples of this test representing a wide spectrum of pharmaceutical substances are enumerated below : S. Stir immediately with a glass rod and opalasecence produced is not allow to stand for 5 minutes protected from light. Limit Test for Sulphates Theory : The limit test for sulphates is based upon its precipitation as barium sulphate in the presence of barium chloride, hydrochloric acid and traces of barium sulphate. The turbidity is not greater than the standard turbidity, when viewed transversely. A few examples of this test consisting of a cross-section of pharmaceutical substances are stated below : S. Limit Test for Arsenate Acetarsol : An organic arsenic compound, being therapeutically active when administered orally, that might be of value in the treatment of spirochaetal or protozoal diseases, for instance : syphilis, yaws, relapsing fever, sleeping sickness and amoebic dysentry. It is made from p-hydroxyphenylarsonic acid, which may be prepared either by straight forward meth- ods from phenol or from p-aminophenylarsonic acid. The resulting compound obtained from either of these reactions is nitrated, reduced and the base is finally acetylated to afford acetarsol. Examples of a few official compounds subject to this test from the Pharmacopoeia are given below : S. Purified Talc When preparing solution A in the test for Calcium, No effervescence produced. Limit Test for Nitrate Basic nitrate is usually found as an impurity in bismuth salts (e. However, this test has a serious disadvantage of correctly matching the yellow colours with great difficulty. Limit Test for Oxalate Oxalate is found to be a frequent impurity in pharmaceutical substances belonging to the category of either organic acids e. The presence of this impurity is due to the following two prime factors, namely : (a) The use of oxalic acid to get rid of Ca2+ during various manufacturing processes. Allow to not more intense than that pro- stand for 2 minutes, decant the liquid into a test- duced by treating 4 ml of a tube containing 0. Sodium Acid Citrate -do- Any red colour produced is not more intense than that pro- duced by treating in the same manner 4 ml of a 0. Com- bine the ethereal layers, evaporate the filtrate to 5 ml and add 1 ml of 2 M acetic acid and l. Limit Test for Phosphate The limit test for phosphate is based upon the formation of a yellow colour reaction with molybdovanadic reagent (combination of ammonium vanadate and ammonium molybdate) in an acidic medium. However, the exact composition of the molybdovanadophosphoric acid complex is yet to be established. Calculate the content of Phosphate from a calibration curve prepared by treating suitable vols. A few typical examples are described below which essentially contains the above cited nonmetallic impurities : 1. The estimation depends upon the conversion of boron to borate and the organic matter is subsequently destroyed by ignition with anhydrous sodium carbonate.
Discount prinivil 10 mg. Blood Pressure Cuff Monitor By DrKea.