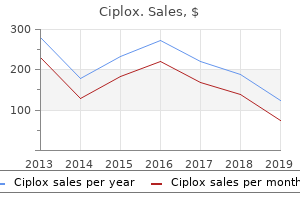
Ciplox
"Buy discount ciplox line, antibiotic resistance gene database".
By: H. Knut, M.B.A., M.D.
Professor, Western University of Health Sciences
For undesirable outcomes antibiotics for dogs at walmart order genuine ciplox on line, a risk ratio that is <1 indicates that the intervention was effective in reducing the risk of that outcome antibiotic 3 days for respiratory infection order cheap ciplox. Antihistamines Page 53 of 72 Final Report Update 2 Drug Effectiveness Review Project Run-in period: Run in period: A period before randomization when participants are monitored but receive no treatment (or they sometimes all receive one of the study treatments antibiotics kill candida order cheap ciplox online, possibly in a blind fashion). The data from this stage of a trial are only occasionally of value but can serve a valuable role in screening out ineligible or non-compliant participants, in ensuring that participants are in a stable condition, and in providing baseline observations. A run-in period is sometimes called a washout period if treatments that participants were using before entering the trial are discontinued. This term (or the term ‘‘safe’’) should not be used when evidence on harms is simply absent or is insufficient. Sample size: The number of people included in a study. In research reports, sample size is usually expressed as "n. Larger sample sizes also increase the chance that rare events (such as adverse effects of drugs) will be detected. Sensitivity analysis: An analysis used to determine how sensitive the results of a study or systematic review are to changes in how it was done. Sensitivity analyses are used to assess how robust the results are to uncertain decisions or assumptions about the data and the methods that were used. Side effect: Any unintended effect of an intervention. Side effects are most commonly associated with pharmaceutical products, in which case they are related to the pharmacological properties of the drug at doses normally used for therapeutic purposes in humans. Standard deviation (SD): A measure of the spread or dispersion of a set of observations, calculated as the average difference from the mean value in the sample. Standard error (SE): A measure of the variation in the sample statistic over all possible samples of the same size. The standard error decreases as the sample size increases. Standard treatment: The treatment or procedure that is most commonly used to treat a disease or condition. In clinical trials, new or experimental treatments sometimes are compared to standard treatments to measure whether the new treatment is better. Statistically significant: A result that is unlikely to have happened by chance. Study: A research process in which information is recorded for a group of people. The data are used to answer questions about a health care problem. Study population: The group of people participating in a clinical research study. The study population often includes people with a particular problem or disease. It may also include people who have no known diseases. Subgroup analysis: An analysis in which an intervention is evaluated in a defined subset of the participants in a trial, such as all females or adults older than 65 years. Superiority trial: A trial designed to test whether one intervention is superior to another. Surrogate outcome: Outcome measures that are not of direct practical importance but are believed to reflect outcomes that are important; for example, blood pressure is not directly important to patients but it is often used as an outcome in clinical trials because it is a risk factor for stroke and heart attacks. Surrogate endpoints are often physiological or biochemical markers that can be relatively quickly and easily measured, and that are taken as being predictive of important clinical outcomes. They are often used when observation of clinical outcomes requires long follow-up. Antihistamines Page 54 of 72 Final Report Update 2 Drug Effectiveness Review Project Survival analysis: Analysis of data that correspond to the time from a well-defined time origin until the occurrence of some particular event or end-point; same as time-to-event analysis. Systematic review: A review of a clearly formulated question that uses systematic and explicit methods to identify, select, and critically appraise relevant research and to collect and analyze data from the studies that are included in the review. The extent to which a drug’s adverse effects impact the patient’s ability or willingness to continue taking the drug as prescribed.
However antibiotics for ear infection order ciplox uk, other studies failed to show any effect on lipoatrophy (Bernadino 2013) virus 87 buy cheap ciplox 500 mg. In MONARK antibiotics for acne sun exposure purchase 500mg ciplox with mastercard, bone density improved after a switch to darunavir/r monotherapy (Guarladi 2014). However, some patients on lopinavir/r show low levels of viremia, especially in combination with low CD4 T cells. They tend to show poor compliance (Campo 2007, Pulido 2008, Gutmann 2010). The same was observed with therapy-naïve patients (see above). For darunavir, the results of two large randomized studies MONET and MONOI with identical design are published (Clumeck 2011, Valentin 2012). In MONET, non-infe- riority of the monotherapy could not completely be shown after 96 weeks, at least regarding the primary endpoints (Clumeck 2011). In total, 82% of patients were below 50 copies/ml in the standard arm at week 96, compared to 78% on darunavir monotherapy. When virologi- cally successful therapies were not evaluated as failure, a difference was not observed. The results can be explained by a possibly low adherence in the mono-arm (with significantly more HCV-coinfected patients). In MONOI, transient viremia was more frequent on monotherapy and a permanent control under 50 copies/ml without blips was observed in 59% versus 70% of patients at week 96 (Valantin 2012). Virologic failure was associated with levels of proviral DNA at baseline (Marcelin 2011), but also with low adherence (Lambert-Niclot 2011). Of note, darunavir RAMs were not observed either in MONET or in MONOI (Lambert- Niclot 2012, Pulido 2012). Possibly, darunavir levels are lower without NRTIs (Garvey 2010). In MONOI, lipoatrophy improved in some patients (Valantin 2012). For indinavir/r, saquinavir/r and fosamprenavir/r there are one-arm pilot studies with weak results (Kahlert 2004, Patricia 2010, Saumoy 2011). The Ataritmo study observed an elevated viral load in cerebrospinal fluid within some patients on atazanavir with otherwise well suppressed viral loads. In the OREY study, 9/63 patients developed virological failure (Pulido 2009). In a systematic review of 10 randomized trials of 1,964 patients with HIV RNA suppression at baseline, PI monotherapy showed a higher risk of HIV RNA eleva- tions, and small numbers with HIV RNA detectable in CSF and concomitantly in the plasma. However, there was no increased risk of treatment-emergent drug resistance (Arribas 2014). The risk of treatment emergent NRTI or PI resistance was 11/973 (1. HIV-1 RNA suppression rates after intensification were similar between PI monotherapy and triple therapy. More recently, new approaches such as dual therapies (ususally with 3TC as a single NRTI) have been evaluated. In studies like SALT and OLE, atazanavir/r or lopinavir/r have been combined with 3TC (see above), the results are encouraging. In contrast, dual therapy with an NNRTI is not recommended. In the COOL study, many patients developed virological failure on TDF plus efavirenz (Girard 2006). Conclusion: Monotherapies with boosted PIs such as lopinavir/r and darunavir/r are slightly less effective than classic therapies (review: Mathis 2011). In most cases, low- level viremia without resistance appears that does disappear upon intensification (Arribas 2014).
Cheap ciplox 500 mg fast delivery. Antimicrobial resistance video.
Human HDACs are classified into 4 classes: oral daily dose of 25 mg on days 1-21 of a 4-week cycle in patients class I includes HDAC 1 bacteria on the tongue buy ciplox once a day, 2 best antibiotic for sinus infection cipro order ciplox 500mg, 3 bacteria icd 9 code order ciplox master card, and 8, which are localized to the with relapsed or refractory HL demonstrated modest single-agent nucleus with ubiquitous tissue expression; class II includes HDAC 36 activity. Thirty-eight patients with a median of 4 prior therapies 4, 5, 6, 7, 9, 10, which have variable cellular localization; class III demonstrated an ORR of 19%, 1 patient with CR, 6 patients with includes NAD-dependent homologs of yeast, SIRT 1-7, which are PR, and 5 patients with stable disease (Figure 2). The most common not being targeted by currently available HDAC inhibitors; and 26 dose-limiting toxicities were cytopenias, rash, and hepatic toxicity. Preclinical and clinical experiments have demonstrated that HDAC inhibitors may have a potential therapeutic value in patients with HL by a direct antitumor Conclusion effect plus an indirect immunoregulatory effect (Figure 3). Since 1977, brentuximab vedotin is the only new which is responsible for T-cell chemotaxis. Many new small-molecule inhibitors have promis- disrupting the interaction between PD-1 and PD-L1/2 interactions, ing clinical activity, paving the way for a revolution in the treatment which are required for the generation of memory T cells and of HL (Figure 2). Although some seem to have direct activity as self-recognition by regulatory T cells. Future investiga- evaluated recently for the treatment of relapsed HL and have shown tions should focus on identifying predictive biomarkers to help encouraging results (Figure 1). Vorinostat is one of the earliest select patients who are more likely to respond to these novel agents. Novartis, Curis, and Johnson and Johnson and has received 13. PI3Kdelta inhibitor, honoraria from Seattle Genetics, Gilead, Novartis, and Sanofi. GS-1101 (CAL-101), attenuates pathway signaling, induces Off-label drug use: Emerging treatment strategies using brentux- apoptosis, and overcomes signals from the microenvironment imab vedotin, PI3K inhibitors, HDAC inhibitors, and ibrutinib in in cellular models of Hodgkin lymphoma. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin Correspondence lymphoma. Anas Younes, Lymphoma Service, Memorial Sloan-Kettering Cancer 15. Johnston PB, Pinter-Brown L, Rogerio J, Warsi G, Chau Q, Center, 1275 York Ave, Box 330, New York, NY 10065; Phone: Ramchandren R. Everolimus for relapsed/refractory classical 212-639-7715; Fax: 646-422-2291; e-mail: younesa@mskcc. Hodgkin lymphoma: multicenter, open-label, single-arm, phase 2 study [abstract]. Vorinostat phase II study of brentuximab vedotin for patients with relapsed inhibits STAT6-mediated TH2 cytokine and TARC production or refractory Hodgkin’s lymphoma. The pan-deacetylase (SGN-35) in patients with transplant-naive relapsed/refractory inhibitor panobinostat induces cell death and synergizes with Hodgkin lymphoma. Published online ahead everolimus in Hodgkin lymphoma cell lines. Safety and efficacy of imab vedotin in CD30-positive hematologic malignancies: a the novel combination of panobinostat (LBH589) and everoli- phase II study [ASCO Annual Meeting Abstracts]. J Clin mus (RAD001) in relapsed/refractory Hodgkin and non- Oncol. SB1518, a novel AFM13 in patients with relapsed or refractory Hodgkin lym- macrocyclic pyrimidine-based JAK2 inhibitor for the treatment phoma [abstract]. The JAK inhibitor rituximab plus ABVD in patients with newly diagnosed classi- AZD1480 regulates proliferation and immunity in Hodgkin cal Hodgkin lymphoma. Oncogenic kinase rituximab-ABVD in classical Hodgkin lymphoma. PD-1-PD-1 refractory Hodgkin or non-Hodgkin lymphoma treated in a ligand interaction contributes to immunosuppressive microenvi- phase Ia/II clinical trial (NCT00670592) [abstract]. Smith SM, Schoder H, Johnson JL, Jung SH, Bartlett NL, 23. Blum KA, Johnson JL, Niedzwiecki D, Canellos GP, Cheson Cheson BD.
GB Virus C Viremia Is Associated With Higher Levels of Double- Negative T Cells and Lower T-Cell Activation in HIV-Infected Individuals Receiving Antiretroviral Therapy antibiotics for uti not sulfa generic 500mg ciplox with visa. J Infect Dis 2012c; 206:1469-1472 Berenguer M antimicrobial bed sheets purchase online ciplox, Terrault NA antibiotics for staph buy ciplox without a prescription, Piatak M, et al. Hepatitis G virus infection in patients with hepatitis C virus infection undergoing liver transplantation. GB virus C genotype 2 predominance in a hepatitis C virus/HIV infected population associated with reduced liver disease. No influence of GB virus C replication on the prognosis in a cohort of HIV-1- infected patients. Mother-to-child transmission of GB virus C in a cohort of women coinfected with GB virus C and HIV in Bangkok, Thailand. JID 2009:227-35 Bjorkman P, Flamholc L, Naucler A, Molnegren V, Wallmark E, Widell A. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. Chang Q, McLinden JH, Stapleton JT, Sathar MA, Xiang J. Expression of GB virus C NS5A protein from genotypes 1, 2, 3 and 5 and a 30 aa NS5A fragment inhibit human immunodeficiency virus type 1 replication in a CD4+ T- lymphocyte cell line. Tropism of Human Pegivirus (formerly known as GB virus C/Hepatitis G virus)and host immunomodulation: insights into a highly successful viral infection. American Journal of the Medical Sciences 1893;10:487-511. Studies on the transmission of human viral hepatitis to marmoset monkeys. Transmission of disease, serial passages, and description of liver lesions. HIV-1 fusion is blocked through binding of GB Virus C E2-derived pep- tides to the HIV-1 gp41 disulfide loop [corrected]. PLoS One 2013;8:e54452 Ernst D, Pischke S, Greer M, Wedemeyer H, Stoll M. No increased incidence for GB-virus C infection in a cohort of HIV-positive lymphoma patients. Int J Cancer 2011;128:3013 Ernst D, Greer M, Akmatova R, et al. Impact of GB virus C viraemia on clinical outcome in HIV-1-infected patients: a 20-year follow-up study. HIV Med 2014;15:245-50 Gutierrez, RA, Dawson GJ, Knigge MF, et al. Seroprevalence of GB virus C and persistence of RNA and antibody. Impact of GB virus type C infection on mother-to-child HIV trans- mission in the Women and Infants Transmission Study Cohort. Beneficial effect of GB virus C co-infection in Human Immunodeficiency Virus type 1-infected individuals. GB virus C/hepatitis G virus infection: a favorable prognostic factor in hiv-infected patients? Synthetic peptides of hepatitis G virus (GBV-C/HGV) in the selection of putative peptide inhibitors of the HIV-1 fusion peptide. Prevalence and clinical significance of GB virus type C/hepatitis G virus coinfection in patients with chronic hepatitis C undergoing antiviral therapy. J Viral Hepat 2011;18:513-7 Jung S, Eichenmuller M, Donhauser N, et al. HIV entry inhibition by the envelope 2 glycoprotein of GB virus C. Kaufman TM, McLinden JH, Xiang J, Engel AM, Stapleton JT. The GBV-C envelope glycoprotein E2 does not inter- act specifically with CD81. No observed effect of GB virus C coinfection in disease progression in a cohort of African woman infected with HIV-1 and HIV-2.