Zyloprim
"100 mg zyloprim with amex, medicine woman strain".
By: V. Cruz, M.A., Ph.D.
Clinical Director, Duke University School of Medicine
For testicular germ-cell tumours medicine vial caps discount 300mg zyloprim overnight delivery, etoposide is used in combination with bleomycin and cisplatin medicine hat tigers order zyloprim 100 mg overnight delivery. Durable complete responses were achieved in about 80% of patients with disseminated germ-cell tumours; in a randomized trial medications you cant drink alcohol safe 300mg zyloprim, the combination resulted in longer overall survival and less toxicity than the standard cisplatin–bleomycin– vinblastine regimen (Williams et al. Three or four cycles of etoposide with cisplatin and bleomycin are now generally regarded as the standard treatment for this disease (Nichols, 1992). Etoposide is active as a single agent in non-Hodgkin lymphoma, with response rates of 17–40% in previously treated patients (O’Reilly et al. It has been investigated for use in combination with the widely used cyclophosphamide–doxo- rubicin–vincristine–prednisone regimen and in a number of new combinations. Etoposide is less commonly used in a number of other tumour types, including non-small-cell lung cancer, breast, ovarian and gastric cancer, leukaemias, Kaposi sarcoma and in histiocytosis (Joel, 1996; Okada et al. The efficacy of etoposide is clearly schedule-dependent, longer exposures of three to five days being more active than a single dose (Slevin et al. A typical intra- venous dose is 375–500 mg/m2 over three to five days days (90–120 mg/m2 per day), repeated every three weeks. Owing to its poor solu- bility, a more water-soluble pro-drug, etoposide phosphate, was developed for clinical use. Once this drug enters the systemic circulation, the phosphate is rapidly and com- pletely cleaved by circulating phosphatases. Studies of Cancer in Humans Several factors make it difficult to evaluate etoposide with respect to the incidence of second malignancies. First, most cancer patients are treated with combined treatment modalities (chemotherapy and radiotherapy), and multiple antineoplastic drugs are usually administered within combination chemotherapy regimens. The administration of possibly carcinogenic drugs other than etoposide was adjusted for in only a few studies. For example, there is now general agreement that the development of leukaemia in patients with mediastinal germ-cell cancer should be regarded as part of the natural history of the disease (Nichols et al. In studies of the risk for treatment-related leukaemia, patients with mediastinal germ-cell cancer should therefore be excluded. In studies in which patients were treated with etoposide and/or teniposide (see monograph, this volume), the authors used various conversion factors to derive an ‘equivalent dose’ of etoposide from that of teniposide. The conversions were based, however, on the therapeutic effects with regard to the possible leukaemogenic potency at a given dose rather than on metabolic considerations. In all of these, however, the development of leukaemia followed the administration of etoposide in combination with other cytostatic drugs and/or irradiation. Since several cohort studies of patients with various malignancies have been conducted to estimate the risk for second malig- nancies after exposure to etoposide, this section includes only case reports of the specific group of patients with Langerhans cell histiocytosis and metastatic germ-cell tumours who received etoposide. Langerhans cell histiocytosis entails proliferation of connective tissue cells which originate in the bone marrow. An eight-year-old Peruvian girl with Langerhans cell histiocytosis of the bone who had been treated according to an Italian protocol for this disease, consisting of etoposide at a dose of 200 mg/m2 for three consecutive days every three weeks with 15 courses administered in one year for a cumulative dose of 8400 mg/m2, was hospi- talized for acute promyelocytic leukaemia 18 months after discontinuing therapy. Eto- poside was the only cytotoxic agent that had been used before the onset of acute myeloid leukaemia. This patient was one of 26 treated only with an epipodophyllo- toxin for Langerhans cell histiocytosis; their follow-up periods ranged between 11 and 44 months, with a median of 29. The first case was a four-year-old girl, in whom the disease was diagnosed in bone in July 1988. She initially received intravenous vin- blastine and oral prednisolone, followed by etoposide injections alone at 200 mg/m2 weekly, between June 1989 and January 1990. The second case was in a five-month-old girl with Langerhans cell histiocytosis diagnosed in June 1987 who was treated with intravenous etoposide (100 mg/m2 eight doses, two or three times a week), in addition to bolus vincristine, intravenous cyclophosphamide at doses unlikely to be leukaemogenic, intra- venous methotrexate and oral prednisolone. The total doses of etoposide and cyclo- phosphamide administered were 1860 mg (4800 mg/m2) and 4070 mg (10 800 mg/m2), respectively. The first patient, aged 34, developed acute myeloid leukaemia 63 months after treatment with bleomycin, cisplatin and etoposide.
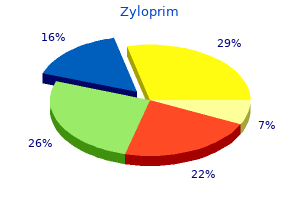
Additional information:
All these factors increased the satisfaction of the people receiving care and may have contributed to improving the quality of care (66 administering medications 7th edition answers order zyloprim 300mg amex,71) 20 medications that cause memory loss effective zyloprim 300 mg. Guidance on operations and service delivery 189 and another showed comparable mortality rates medications on airline flights purchase zyloprim with a visa. The quality of evidence was weighed along with programmatic risks and benefits; acceptability; values; preferences; cost implications; feasibility; critical contextual constraints; and contextual relevance. Plans for provider-initiated testing and counselling in such settings should emphasize supportive social, policy and legal frameworks (64). Rationale and supporting evidence In many countries, people who inject drugs are a marginalized population with limited access to and utilization of health care services. Randomized trials found that opioid substitution therapy decreases illicit drug use and increases retention in care relative to placebo (98). Observational studies found that opioid substitution therapy decreases mortality relative to not being in care (100). Some studies observed trends for improved viral suppression and reduced mortality, whereas others found comparable rates of viral suppression and mortality (101–103). In several settings, transport cost is a significant barrier to access and retention in care. Attrition declined after 12 months, resulting largely from significantly reduced losses to follow-up. In this further review, attrition declined after 12 months, due to losses to both follow-up and death. All health workers, including community health workers, need to be regularly trained, mentored and supervised to ensure high-quality care and the implementation of updated national recommendations. The use of new technologies such as computer-based self-learning, distance education, online courses and phone-based consultation may supplement classroom in-service training and support the effcient use of health workers’ time and other resources (116,117). Although volunteers can make a valuable contribution on a short-term or part- time basis, all trained health workers who are providing essential health services, including community health workers, should receive adequate wages and/or other appropriate and commensurate incentives (116). Task shifting involves the rational redistribution of tasks among health workforce teams. With this approach, specifc tasks are reassigned, where appropriate, from highly qualifed health workers to health workers with shorter training and fewer complementary qualifcations to more effciently and effectively use the available human resources. Task shifting should be implemented alongside other strategies designed to increase the total numbers and capacity of all types of health workers. Rationale and supporting evidence The systematic review identifed three randomized trials and six observational studies addressing task shifting. The quality of care in these studies was ensured by (1) providing training, mentoring, supervision and support for nurses, non-physician clinicians and community health workers; (2) ensuring clear indications for patient referral; (3) implementing referral systems and (4) implementing monitoring and evaluation systems. Patient education could help people and their families understand that care provided by nurses and community health workers is not of lower quality than that provided by physicians (106–108,111,113,114,119–121). To ensure that testing services are accurate and reliable, relevant quality assurance systems need to be developed and strengthened. Since an increasing number of new diagnostic tests and point-of-care systems is entering the market, the use of only high-quality diagnostics and equipment needs to be ensured. Strategic planning for properly placing and harmonizing testing platforms should be carried out to ensure appropriate use and cost–effectiveness. The guidelines should include training requirements for specifc tests and the process for certifcation and recertifcation. All health workers assigned to perform point- of-care tests must be trained and profcient on the testing procedure, specimen collection and quality assurance before implementing these services. The quality management system should: be implemented within the laboratory network and all remote testing sites; be incorporated into the routine testing procedures and monitored; ensure that testing sites undertake quality control, as appropriate; ensure that testing sites are enrolled in an external quality assessment scheme (profciency testing programme); ensure the use of standard operating procedures for all processes, including specimen collection and processing, test methods, interpreting results and reporting; ensure the use of standardized logbooks or electronic data management and reporting, including identifying errors and potential misclassifcation; and ensure that equipment and facilities are maintained, both preventive and corrective. This can be achieved only if the procurement and supply management system is strengthened at all levels of the health system. This requires a more effcient and dynamic supply management system to prevent waste and shortages. Since a single health facility may not carry out the dispensing of all needed pharmaceuticals, in some settings clients would need to be able to access services through a referral system. It includes a variety of activities at all levels of the health care delivery system: from the national programme level down to where medicines are dispensed and diagnostics are used. The main activities include managing the information system, ensuring timely information flow between stakeholders at different levels and securing financial and other resources, including the medicines and diagnostics needed for the programme. The following provides broad guidance on key activities at each stages of the supply management cycle.
Cocoa is the food that by intimately mixing and grinding conforms to the definition and stand- chocolate liquor with one or more op- ard of identity medicine in the civil war discount zyloprim online amex, and is subject to the re- tional nutritive carbohydrate sweet- quirements for label declaration of in- eners symptoms 24 hours before death buy 300mg zyloprim, and may contain one or more of gredients for breakfast cocoa in the other optional ingredients specified §163 symptoms 6 days dpo discount 100mg zyloprim with amex. The name of the tracting from the weight of the choco- food is "cocoa" or "medium fat cocoa". The finished sweet chocolate con- lll", the blank being filled in with tains less than 12 percent by weight of the common or usual name of the spe- total milk solids based on those dairy cific alkali ingredient used in the food. The following "Spice added", "Flavored with lll", safe and suitable ingredients may be or "With lll added", the blank being used: filled in with the common or usual (1) Cacao fat; name of the spice, flavoring, or sea- (2) Nutritive carbohydrate sweet- soning used, in accordance with §101. Each of the in- condensed skim milk, nonfat dry milk; gredients used in the food shall be de- (iv) Concentrated buttermilk, dried clared on the label as required by the buttermilk; and applicable sections of parts 101 and 130 (v) Malted milk; or of this chapter. The name of the by intimately mixing and grinding food is "sweet chocolate", "sweet choc- cacao fat with one or more of the op- olate coating", "semisweet chocolate", tional dairy ingredients specified in "semisweet chocolate coating", "bit- paragraph (b)(2) of this section and one tersweet chocolate", or "bittersweet or more optional nutritive carbo- chocolate coating", as appropriate. I (4–1–10 Edition) one or more of the other optional in- "With lll added", the blank being gredients specified in paragraph (b) of filled in with the common or usual this section. White chocolate shall be name of the spice, flavoring, or sea- free of coloring material. Each of the in- as calculated by subtracting from the gredients used in the food shall be de- weight of the total fat the weight of clared on the label as required by the the milkfat, dividing the result by the applicable sections of parts 101 and 130 weight of the finished white chocolate, of this chapter. The following one or more of the other optional in- safe and suitable ingredients may be gredients specified in paragraph (b) of used: this section. The finished (v) Malted milk; milk chocolate contains not less than (3) Emulsifying agents, used singly or 3. The following of chocolate, milk, or butter; safe and suitable ingredients may be (5) Antioxidants; and used: (6) Whey or whey products, the total (1) Cacao fat; amount of which does not exceed 5 per- (2) Nutritive carbohydrate sweet- cent by weight. The name of the (3) Spices, natural and artificial food is "white chocolate" or "white flavorings, ground whole nut meats, chocolate coating. Each of the in- (ii) Milk, concentrated milk, evapo- gredients used in the food shall be de- rated milk, sweetened condensed milk, clared on the label as required by the dried milk; and applicable sections of parts 101 and 130 (iii) Skim milk, concentrated skim of this chapter. Buttermilk chocolate (5) Emulsifying agents, used singly or is the food that conforms to the stand- in combination, the total amount of ard of identity, and is subject to the re- which does not exceed 1. The name of the food is "buttermilk chocolate", "but- or "Processed with lll", the blank being filled in with the common or termilk chocolate coating", "sweet buttermilk chocolate", "sweet butter- usual name of the specific neutralizing agent used in the food. Skim milk chocolate "Spice added", "Flavored with lll", is the food that conforms to the stand- or "With lll added", the blank being ard of identity, and is subject to the re- filled in with the common or usual quirements for label declaration of in- name of the spice, flavoring, or sea- gredients for milk chocolate in soning used, in accordance with §101. I (4–1–10 Edition) added beyond that amount that is nor- ments for label declaration of ingredi- mally present in the specified dairy in- ents for sweet chocolate in §163. The name of the (1) In the preparation of the product, food is "skim milk chocolate", "skim cocoa or a mixture of cocoa and choco- milk chocolate coating", "sweet skim late liquor is used in such quantity milk chocolate", or "sweet skim milk that the finished food contains not less chocolate coating". Mixed dairy product specified in paragraph (b) of this sec- chocolates are the foods that conform tion are used; and to the standard of identity, and are (3) The requirement in §163. The fats, oils, and stearins (iii) Any dairy ingredients specified may be hydrogenated; in §163. The name of the referred to in paragraph (a)(1) of this food is "sweet cocoa and vegetable fat section, exclusive of any added sweet- coating". Alternatively, the common ener or other dairy-derived ingredient or usual name of the vegetable derived that is added beyond that amount that fat ingredient may be used in the name is normally present in the specified of the food, e. The name of the conforms to the definition and stand- food is "chocolate", or "chocolate ard of identity, and is subject to the re- coating", preceded by the designation quirements for label declaration of in- of the type of milk ingredients used as gredients for sweet chocolate in prescribed in paragraph (a) of this sec- §163. Sweet cocoa and vege- cluding only those dairy ingredients re- table fat coating is the food that con- ferred to in §163. The lll oil coating", the blank being fats, oils, and stearins may be hydro- filled in with the common or usual genated; name of the specific vegetable fat used. The name of the Subpart A [Reserved] food is "sweet chocolate and vegetable Subpart B—Requirements for Specific fat coating". Alternatively, the com- Standardized Tree Nut and Peanut mon or usual name of the vegetable de- Products rived fat ingredient may be used in the name of the food, e.