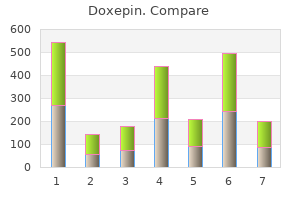
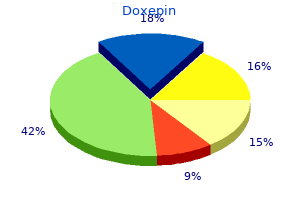
Doxepin
"Purchase cheap doxepin, anxiety 40 weeks pregnant".
By: H. Goose, M.B.A., M.B.B.S., M.H.S.
Associate Professor, Louisiana State University
The most straightforward application is in cancer treatment anxiety symptoms 4dp3dt purchase discount doxepin on-line, with several products (Table 1) in market such as Caelyx r anxiety grounding cheap doxepin 10 mg line, Doxil r anxiety symptoms vertigo buy doxepin with a visa, Transdrug r , Abraxane r , etc. In 1904, Paul Ehrlich (1854–1915), one of the great architects of medical sci- ence, published three articles in the Boston Medical and Surgical Journal, the imme- diate predecessor of the New England Journal of Medicine. These articles, which concerned Ehrlich’s work in immunology, were summaries of the Herter lectures he had given at Johns Hopkins University. They dealt with immunochemistry, the mechanism of immune hemolysis in vitro, and the side-chain theory of antibody formation. At the time of the Herter lectures, Ehrlich was at the peak of his intellec- tual powers and scientific influence. He was not only the father of hematology but also one of the founders of immunology. He made key contributions in the field of infectious diseases and, with his idea of the “magic bullet,” initiated a new era of chemotherapy (4). The concept of Paul’s magic bullet has turned out to be a reality with the approval of several forms of drug-targeting systems for the treatment of certain cancer and infectious diseases. In most cases, either polymers or lipids are used as carriers for the drug, and the delivery systems have particle size distribution from few nanometers to few hundred nanometers (Table 2). New and newer polymers have been tried to develop nanoparticles for their application as drug carriers. Various parameters such as particle size distribution, dimensional analysis, and zeta-potential were used to characterize these systems. These nanosystems were evaluated for cell compatibility, and their ability to escape phagocytosis was also characterized. Protein-based nanoparticulate drug delivery systems are defined as pro- teins being biodegradable, biocompatible, very versatile molecules, and can be used as drug carriers (28). A protein-based nanoparticulate drug delivery system is already in the market (paclitaxel-loaded albumin nanoparticles, Abraxane r ). Protein macromolecules offer many advantages over their synthetic counterparts (synthetic polymers that are commonly used as drug carriers). Terminologies used (nm) References Polymeric systems 1 Dendrimers 1–10 1,5 2 Polymer micelles 10–100 1 3 Niosomes 10–150 1 4 Nanoparticles 50–500 1,6–10 5 Nanocapsules 100–300 1,11,12 6 Nanogels 200–800 1 7 Polymer–drug nanoconjugates 1–15 13–16 8 Chitosan polymers 100–800 17,18 9 Methacrylate polymers 100–800 19 Lipid systems 1 Solid lipid nanoparticles 50–400 20 2 Lipid nanostructured systems 200–800 21 3 Cubosomes 50–700 1 4 Liposomes 10–1000 22 5 Polymerosomes 100–300 13 6 Immunoliposomes 100–150 13 Protein/peptide nanotubes 1 Peptide nanotubes 1–100 23 2 Fusion proteins and immunotoxins 3–15 13 Metal nanostructures 1 Metal colloids 1–50 1,9 2 Carbon nanotubes 1–10 (diameter) 1 and 1–1000 (length) 3 Fullerene 1–10 1 4 Gold nanoparticles 100–200 13,24 5 Gold nanoshells 10–130 13 6 Silicone nanoparticles 25 7 Magnetic colloids 100–600 26 when used as drug carriers. Owing to this property, these can be used for deliv- ering different drug molecules. As these protein molecules are biocompatible and biodegradable, this is a distinct advantage over their synthetic counterparts. Some of the natural organic and protein molecules are also described as carriers for drug. These are fabricated as nanoparticles or nanofibers for delivering the drugs (29–31). Gregory Gregoriadis in 1974 (32) lead to several breakthrough discoveries by using nanoparticles as drug carriers resulting from cutting-edge researches based on multidisciplinary approaches, many more applications have developed. We have 4 Pathak discussed in detail about the nanoparticulate drug delivery systems in our first vol- ume in chapters 1 and 13, which covered most of the development and technologies and applications till 2005. Several research reports have been published on the applications of nanoparticulate drug delivery systems using various drug entities and polymers and different forms of drug delivery systems. The employment of poly(butyl cyanoacrylate) nanoparticles showed high efficacy of nanoparticle-bound doxoru- bicin in intracranial glioblastoma in rats. An interest- ing review on the application of nanotechnology in breast cancer therapy is covered by Tanaka et al. More than 150 clinical trials are being conducted worldwide for the treat- ment of breast cancer by using nanotechnology-based products. This review covers different generations of nanotechnology tools used for drug delivery, especially in breast cancer. Injectable drug delivery nanovectors are used for cancer therapy, especially when multiple-drug therapy is used. These vectors need to be large enough to evade the body defense but should be sufficiently small to avoid blockages in even the capillaries. As these vectors are smaller than the diameters of the capillaries, the blockages can be effectively prevented (13).
They studied the ability of four paid volunteer male college students to learn lists of adjectives after twenty-four and forty-eight hours of confinement anxiety symptoms for xanax discount doxepin 75 mg. Comparing their experimental subjects to an equivalent control group anxiety symptoms in young adults cheap doxepin, they found that the ability at rate-learning improved with continued sensory deprivation anxiety girl cartoon order doxepin 10 mg with visa. In a follow-up study, nine experimental and nine control subjects, who were all paid volunteer male college students, were compared for ability to learn a longer list of adjectives after twenty-four, forty-eight, and seventy- two hours of sensory deprivation (77). In this instance there were no significant differences between groups in errors or trials to criterion, although -64- the experimental group made fewer overt errors and showed less variability. Thus, despite failure to confirm their own previous findings, this study did not support the deterioration finding of the McGill group. Goldberger and Holt (32) studied fourteen paid volunteer male college students under perceptual deprivation conditions similar to those of the McGill experiments. Subjects lay on a bed in a cubicie for eight hours and were encouraged to talk during their time in isolation. The following tests were administered at the end under the experimental conditions: arithmetic reasoning, digit span, and story recall. Subjects were then taken out of the isolation and a test of logical deductions was given. Comparison of the performance of the experimental subjects pre- and postconfinement (without a control group) showed that only the last of these, logical deductions, reflect significant impairment. Davis, McCourt, and Solomon (21) utilizing a modification of the polio tank- respirator procedure initially described by Wexler et al. Although they could talk to each other, they were confined separately and could not see each other. In comparing scores before and after isolation they found no change in performance on a block design task. These authors considered the possibility of procedural variables causing failure to confirm Bexton et al. Subjects were seated individually for one hour in an isolation chamber in a comfortable chair. They wore goggles which were either blacked out or else permitted diffuse light perception. Audition was minimized through car plugs, padded earphones, and the masking sound of a fan motor. Their fingers were wrapped in elastic bandages and they wore elbow- length gloves. Subjects were also told that they would perceive sensations ordinarily below conscious awareness. These experimenters report that there was no "gross cognitive deterioration" under these conditions as measured by the number of word associations produced in two minutes. The small sample size, the brief period of isolation, and the limited measure employed in this study suggests caution in interpreting this result. Cohen, Silverman, Bressler, and Shmavonian (18) reported an exploratory investigation on four subjects exposed singly to four hours of confinement and deprivation while seated in an anechoic chamber, with instructions to keep awake and to estimate the passage of successive thirty-minute intervals. All four subjects showed an increase in performance on digit span, and decrease in arithmetic reasoning, abstraction, and general reasoning. The small sample size and absence of a control group limit the relevance of these findings. The few reports available, their currently sketchy detail, and their limited controls make it difficult to arrive at a firm generalization concerning the effects of deprivation and isolation on cognitive skills. It appears that the skill most severely impaired under these conditions is that of general reasoning and problem solving, whether the situation involves verbal-conceptual materials or numbers. On the other hand, in several studies performance on simple recall tasks or rote learning seems either to improve or else does not decline. Tasks that involve analysis and synthesis of visual materials such as block design show equivocal results; in some studies there is deterioration, in others no change is seen.
Purchase doxepin 25 mg line. 5 ways to make friends with social anxiety.
Compatible Diluents/Administration Captopril is only available for oral/enteral administration anxiety of influence 75mg doxepin for sale. Administer via an infusion over 5 minutes Infants/children: Oral anxiety symptoms relief purchase doxepin 25 mg without prescription, enalapril: initial or “test” dose 0 anxiety symptoms centre generic doxepin 25mg on line. Administer via an infusion over 5 minutes Adults: Oral, enalapril: initial or “test” dose 2. Maximum dose, 5 mg/dose every 6 hours (20 mg/day) Note: Dosing for all age groups should be titrated to an individual patient’s response, and the lowest dose that achieves this response should be cho- sen. For additional dosing precautions in neonates, see “Poisoning Information” Dosing adjustment for renal impairment: Cl 10 to 50 mL/min/1. Moni- toring for blood pressure effect should focus on the period 1 to 3 hours (enal- april) or 15 to 60 minutes (enalaprilat) after dosing. Adverse Effects Cardiovascular: hypotension, tachycardia, syncope Respiratory: cough, dyspnea, eosinophilic pneumonitis. Central nervous system: fatigue, vertigo, dizziness, headache, insomnia Gastrointestinal: nausea, diarrhea, loss of taste perception Hepatic: cholestatic jaundice, fulminant hepatic necrosis (rare, but poten- tially fatal) Renal: diminished renal function Genitourinary: impotence Neuromuscular and skeletal: muscle cramps Endocrine/metabolic: hypoglycemia, hyperkalemia Hematological: agranulocytosis, neutropenia, anemia Cutaneous/peripheral: rash, angioedema. The risk of angioedema is higher in the first 30 days of use and for enalapril and lisinopril as compared with captopril Drug-Drug Interactions In patients who are also receiving potassium supplements or a potassium- sparing diuretic (e. Poisoning Information Enalaprilat contains benzyl alcohol (9 mg/mL), which may cause allergic reac- tions and a potentially fatal toxicity in neonates, called “gasping syndrome” at high doses (≥ 99mg/kg/d). Gasping syndrome is manifested by metabolic acidosis, respiratory distress with gasping respirations, central nervous system dysfunction (seizures, hemorrhage), hypotension, and cardiovascular collapse. Therefore, enalaprilat should be used with caution and close monitoring in neonates. Compatible Diluents/Administration Enalapril is available for oral/enteral administration. Enalaprilat can be administered undi- luted or diluted with normal saline; infuse over 5 minutes. Dosing Neonates (premature and full term), infants, and children younger than 6 years: no dosing information is available; because of this, the manufacturer recommends not using lisinopril in patients younger than 6 years of age Children older than 6 years: initial or “test” dose 0. Increase dose by at most 10mg/dose by at least 2-week intervals based on clinical response. Maximum dose is 40 mg/day Note: Dosing for all age groups should be titrated to an individual patient’s response, and the lowest dose that achieves this response should be cho- sen. For additional dosing precautions in neonates, see “Poisoning Information” Dosing adjustment for renal impairment: Cl greater than 30 mL/min/1. Monitoring for blood pressure should be conducted with knowledge that the maximum effect is 6 to 8 hours after dosing. The risk of neutropenia is increased in patients with renal dysfunction Cutaneous/peripheral: rash, angioedema. The risk of angioedema is higher in the first 30 days of use and is greater for lisinopril and enalapril than captopril Other: anaphylactoid reactions Precautions Note: Dosing for all age groups should be titrated to an individual patient’s response, and the lowest dose that achieves this response chosen. Airway obstruction can occur with swelling of the tongue, larynx, or glottis, especially in patients who have a history of airway surgery. For patients at higher risk of airway obstruction, equipment to establish air- way patency and medications to relieve airway swelling (e. Drug-Drug Interactions In patients who are also receiving potassium supplements or a potassium-sparing diuretic (e. Compatible Diluents/Administration Only available for oral/enteral administration. In pediatric patients, it is used predominantly to treat systemic hypertension, and it seems to have a protective effect on the kidneys in children with renal insufficiency and hypertension. Dosing Neonates (premature and full term) and infants: no data are available to guide dosing in neonates, infants, and children younger than 6 years of age Children 6 to 16 years: Oral: data from a single trial of pediatric patients (n = 177) aged 6 to 16 years forms the basis of pediatric dosing recommendations. Patients receiving diuretics or with low intravascular volume: initial dose, 25 mg once daily. Hepatic impairment: reduce initial dose in adults to 25 mg/d and administer in two versus one dose per day. Pharmacokinetics Onset of action: 6 hours Absorption: well absorbed; bioavailability, 25 to 33% Distribution: volume of distribution—losartan, 34 L; E-3174, 12 L Maximum effect: peak concentrations—losartan, 1 hour; E-3174, 3 to 4 hours Half-life: losartan, 1. Adverse Effects Cardiovascular: chest pain, hypotension, orthostatic hypotension, first-dose hypotension, tachycardia Respiratory: cough, bronchitis, upper respiratory infection, nasal congestion, sinusitis Central nervous system: fatigue, dizziness, hypoesthesia, insomnia Gastrointestinal: diarrhea, gastritis, weight gain, dyspepsia, abdominal pain, nausea Genitourinary: urinary tract infection (patients with diabetic nephropathy) Neuromuscular and skeletal: weakness, back pain, knee pain, leg pain, muscle cramps, myalgia Endocrine/metabolic: hypoglycemia, hyperkalemia Hematological: anemia Cutaneous/peripheral: cellulitis (patients with diabetic nephropathy) Other: fever, infections, flu-like syndrome Precautions Drugs that affect the angiotensin system in humans can cause injury or death to a fetus during the second or third trimester; therefore, losartan should be dis- continued as soon as possible once pregnancy is detected. Because losartan can cause hypotension, especially with the initial dose, particular care should be used in patients who have low intravascular volume.
Adverse Efects Adrenal suppression; immunosuppression; anaphylaxis; musculoskeletal pain; depression; fatigue; sinusitis; oropharyngeal infections; upper respiratory tract infection; gastrointestinal disturbances; conjuctivitis; otitis media; local irritation and sensitization; bacterial skin infection; skin depigmentation; cataract; growth suppression anxiety when trying to sleep order doxepin once a day. Child- 2-5yrs: 4 mg once daily; 6-14 yrs: 5 mg once daily; ≥ 15 yrs: 10 mg once daily anxiety symptoms heart pain best doxepin 25 mg. Adverse Efects Headache; rashes; eosinophilia; neuropathy; Churg-strauss syndrome anxiety 2 buy genuine doxepin online. Salbutamol* Pregnancy Category-C Schedule H Indicatons Prophylaxis and treatment of asthma; premature labour; reversible airway obstructon. Dose Oral Adult- Chronic asthma (when inhalaton is inefectve): 2 to 4 mg, 3 or 4 tmes daily; in some patents up to max. Child- Chronic asthma (when inhalaton is inefectve): under 2 years; 100 µg/kg, 4 tmes daily. Slow intravenous injecton Adult- Severe acute bronchospasm: 250 µg, repeated if necessary. Child- Relief of acute bronchospasm: 100 µg (1 puf) increased to 200 µg (2 pufs); if necessary. Aerosol inhalaton Adult- Prophylaxis of exercise-induced bronchospasm: 200 µg (2 pufs). Chronic asthma (as adjunct in stepped treatment): 100 to 200 µg (1 to 2 pufs), up to 3 to 4 tmes daily. Child- Prophylaxis of exercise-induced bronchospasm: 100 µg (1 puf) increased to 200 µg (2 pufs); if required. Chronic asthma (as adjunct in stepped treatment): 100 µg (1 puf) 3 to 4 tmes daily, increased to 200 µg (2 pufs) 3 to 4 tmes daily; if necessary. Inhalaton of nebulized soluton Adult- Severe acute asthma or chronic bronchospasm unresponsive to conventonal treatment: 2. Child- Severe acute asthma or chronic bronchospasm unresponsive to conventonal treatment, over 18 months: 2. Under 18 months: clinical efcacy uncertain (transient hypoxaemia may occur- consider oxygen supplementaton). Contraindicatons β2agonists are contraindicated in cardiac disease; antepartum haemorrhage; intrauterine infecton; intrauterine fetal death; placenta praevia; abrupto placenta; threatened miscarriage; cord compression; eclampsia or severe pre-eclampsia; diabetes mellitus; thyrotoxicosis. Adverse Efects Hypokalaemia afer high doses; arrhythmias; tachycardia; palpitatons; peripheral vasodi- laton; fne tremor (usually hands); muscle cramps; headache; insomnia; behavioural disturbances in children; hypersensitvity reactons including paradoxical bronchos- pasm; urtcaria and angioedema; slight pain on intramuscular injecton. Storage Store protected from light and moisture at a temperature not exceeding 30⁰C. Dose Oral Adult- Chronic asthma (as tablets): 100 to 200 mg, 3 to 4 tmes daily afer food. Nocturnal asthma (as modifed-release tablets): total daily requirement as single evening dose. Child- Chronic asthma (as tablets); over 12 years: 100 to 200 mg, 3 to 4 tmes daily afer food. Child- Acute severe asthma; by slow intravenous injecton (over at least 20 min): 5 mg/kg. Note: Patents taking oral theophylline (or aminophylline) should not normally receive intravenous aminophylline unless plasma-theophylline concentraton is available to guide dosage and vice versa. Contraindicatons Porphyria; known hypersensitvity to ethylenediamine (for aminophylline). Precautons Cardiac disease; hypertension; hyperthy- roidism; peptc ulcer; epilepsy; hepatc impairment; pregnancy (Appendix 7c); lacta- ton (Appendix 7b); elderly; fever; smokers may require larger or more frequent doses; interactons (6b, 6c). Adverse Efects Nausea vomitng and other gastrointestnal disturbances; restlessness; anxiety; tremor; palpitatons; headache; insomnia; dizziness; convulsions; arrhythmias and hypotension- especially if given by rapid injecton; urtcaria; erythema and exfoliatve dermatts-resultng from hypersensitvity to ethylenediamine component of aminophylline; neurotoxicity; hypokalemia; metabolic acidosis; gastrointestnal haemorrhage. It is helpful in the expulsion of respiratory secreton and other foreign partcles from respiratory tract. Non-productve cough should be suppressed, whereas productve cough should not be suppressed.